Briefly Describe the Significance of Mendeleev's Periodic Table
The periodic table is also referred to as Mendeleev periodic table. Mendaleevs periodic table contains about 66 elements.

5 Achievements And Limitations Of Mendeleev S Periodic Table Science Laws
Answer to Briefly describe the significance of Mendeleevs periodic.

. A gas in a rigid container remains at constant _______ even if the pressure andor temperature are changed. The periodic table that he introduced in 1869 was a monumental achievementa wonderful mnemonic and a tool that serves to organize the whole of chemistry. In the modern periodic table elements are arranged in order of atomic number in periods and groups.
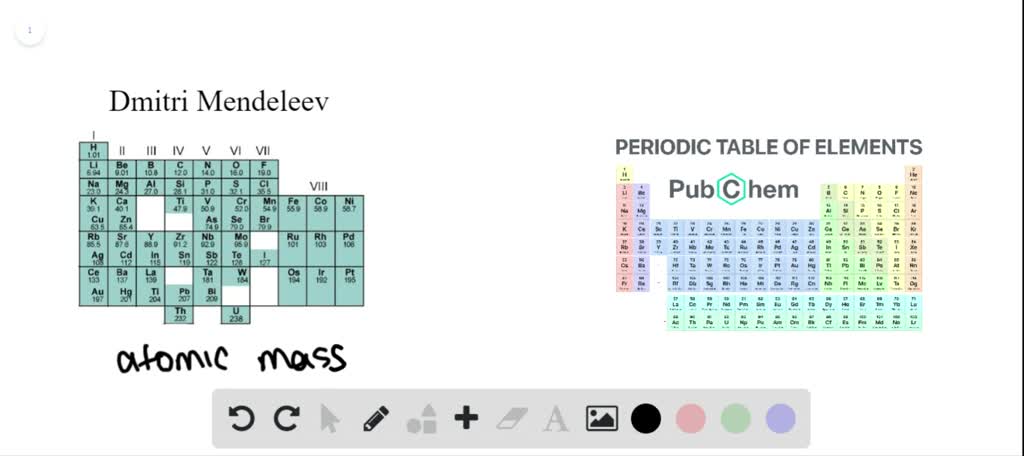
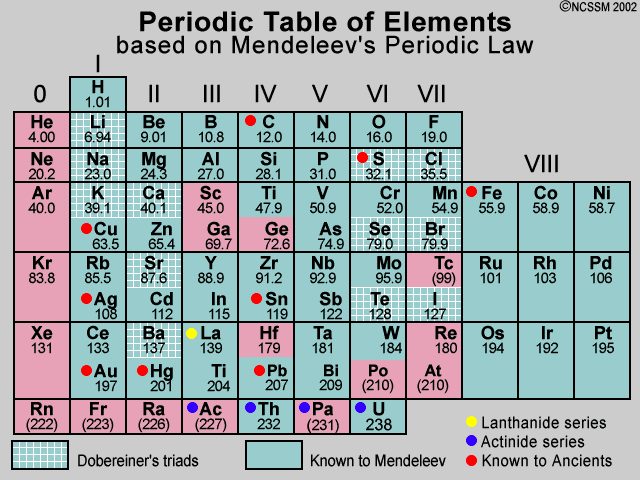
Features of Mendeleevs tables Mendeleev arranged the elements in order of increasing relative atomic mass. Main features of Mendeleevs Periodic Table. Mendeleev had kept some blank spaces for the elements which had yet to be discovered in the place of periodic table.
Periodic table I went this allowed to do Amanda Love to do is by organizing elements into our classic periodic table. C There are eight vertical columns in the periodic table. Periodic table I went this allowed to do Amanda Love to do is by organizing elements into our classic periodic table.
In 1869 a Siberian chemist named Dmitri Mendeleev invented the Periodic Table of Elements. The pressure exerted by the gas will _________ as its temperature increases. See my book Eric Scerri The Periodic Table Its Story and Its Significance OUP 2007.
By Eric Scerri April 12 2007. Looks like this with ah different columns and rows. The periodic table is important as it is so well organized that it provides a lots of information about elements and how they related to one another Figure 5.
Modern periodic table is based on atomic number. Mendeleev successfully predicted Sc Ga and Ge and a further 5 or so elements. Up to 24 cash back This website covers the development of the Periodic Table focusing predominantly on Dmitri Mendeleevs impact on the table.
The law states that the physical and chemical properties of elements are periodic function of their atomic weights. Invest significance of mental Libyans. Briefly describe the significance of Mendeleevs periodic table.
This is shown in Fig 3 a Table from the 1905 edition of Mendeleevs textbook Osnovy ximii3. B grouping of similar elements together. By the way the study of elements became quite simpler as if properties of one element in a particular group were known properties of others could easily be assumed.
The traditional response to this question is that Mendeleev made successful predictions concerning missing elementsblank spaces in his periodic table. Many of us know it simply as the periodic table. Student Study Guide for Chemistry 11th Edition Edit edition Solutions for Chapter 8 Problem 1P.
For many years scientists had been working to find patterns between elements and ingenious ways to explain the patterns. Find step-by-step Chemistry solutions and your answer to the following textbook question. Similar chemical properties of the elements in the same column.
A There are seven horizontal rows in the periodic table. So in this question were asked to briefly describe Mendola. This year marks the 100th anniversary of the death of one of the most famous scientists of all time the Russian chemist Dmitri Ivanovich Mendeleev 18341907.
More importantly he put the elements in their right places in his periodic table correctly pointing out that their atomic. Please navigate using the tabs above. Mendeleev published his first periodic table of the elements in 1869.
Mendeleevs periodic table is based on atomic mass. This is the periodic table we all recognise today. Looks like this with ah different columns and rows.
He gave a law known as periodic law. Mendeleev left space for the elements yet to be discovered. The genius of Mendeleevs periodic table 2012 by Lou Serico TED Ed 424 min.
Mendeleev made an early periodic table. In his table all the elements which have been identified are organized based on mass chemical properties and fundamental properties of an element. Importance of Mendeleevs Periodic Table.
You have probably seen the periodic table before. So in this question were asked to briefly describe Mendola. Dmitri Ivanovich Mendeleev 1834 1907 was a Russian chemist most famous for his contributions to the Periodic TableHe was the first to publish a periodic table similar to the one we use today and is credited for discovering the Periodic law.
Groups like that going across like that and down here its going in. Get solutions Get solutions Get solutions done loading Looking for the textbook. B In a given period there is a regular gradation in the properties of elements from left to right.
Prediction of new elements and their properties. The key difference between his arrangement of the elements and that of Meyer and others is that Mendeleev did not assume that all the elements had been discovered in fact only about two. Mendeleevs periodic table is more accepted for its originality and boldness in making corrections in the properties and predicting unknown elements.
Groups like that going across like that and down here its going in the. The particles of two gases at the same temperature have the same average speed. Solutions for Chapter 8 Problem 1P.
Mendeleevs Periodic table shows that elements with comparable characteristics are arranged in columns at regular intervals. Mendeleev who first published his periodic table in 1869 Figure PageIndex1 is usually credited with the origin of the modern periodic table. Mendeleev was the first one to establish a systematic arrangement and bring out the periodicity of properties of elements with atomic weight and propose a periodic law.
The Group of inert gases is nicely separated to the left. Briefly describe the significance of Mendeleevs periodic table. Invest significance of mental Libyans.
Modern periodic table contains presently 118 elements. In 1869 Mendeleev made remarkable contribution to the classification of elements. Briefly describe the significance of Mendeleevs periodic table.
Mendeleevs periodic table was developed in accordance to-a increasing atomic masses. They are called periods and are numbered from 1 to 7. It allowed the incorporation of these new mysterious gaseous elements as Group 0 after Group VII the halogens and before Group I.
It may even be hanging on the wall of your chemistry class. However when I hope everything is well. The Periodic System now showed its power and flexibility.
In Mendeleevs periodic table there were gaps for undiscovered elementsModern periodic table maintains uniformity. On the basis of this periodic law he arranged all the known elements in the form of table known as periodic table.

Mendeleev Periodic Table Pdf Consists Of Introduction Properties Merits Demerits

Chemistry International Newsmagazine For Iupac

Mendeleev S Periodic Table Charts At Rs 150 Piece Daryaganj New Delhi Id 7575191230
Solved Explain How Mendeleev S Periodic Table Was In Error

Mendeleev S Periodic Table Classification Of Elements Embibe

What Were The Two Criteria Used By Mendeleev In Creating His Periodic Table

Differentiate B W Mendeleev Peridic Table And Modern Periodi Scholr

Solved Describe The Significance Of Mendeleev S Periodic Chegg Com

Did Mendeleev Include Noble Gases In His Periodic Table Quora

What Are The Demerits Of Mendeleev Periodic Table Brainly In

Importance Of Mendeleev S Periodic Table Broad Learnings

Mendeleev S Periodic Table Notes Videos Qa And Tests Grade 11 Chemistry Periodic Classification Of Elements Kullabs

Fragment Of Mendeleev S Periodic Table Of 1904 Showing The Positions Of Download Scientific Diagram

Pdf Mendeleev And The Periodic Table Of Elements

Importance Of Mendeleev S Periodic Table Broad Learnings

Mendeleev S Periodic Table Chemistry For Non Majors

From The Mendeleev Periodic Table To Particle Physics And Back To
Did Mendeleev Include Noble Gases In His Periodic Table Quora
Comments
Post a Comment